- ›
- Why Theracycle ›
- Research and Studies ›
- Forced Exercise: Improving Motor Function in Parkinson’s
Research and Studies
Forced Exercise: Improving Motor Function in Parkinson’s
Alberts, Jay L.; Linder, Susan M.; Penko, Amanda L.; Lowe, Mark J.; Phillips, Micheal
Exercise and Sport Sciences Reviews: October 2011 – Volume 39 – Issue 4 – p 177-186 doi: 10.1097/JES.0b013e31822cc71a
Abstract
Forced exercise has resulted in neuroprotective effects and improved motor function in animal studies. These promising results have not yet been translated fully to humans with Parkinson’s disease (PD), as traditional exercise interventions have not yielded global improvements in function. A novel forced exercise intervention is described that has resulted in improved motor function and central nervous system function in PD patients.
INTRODUCTION
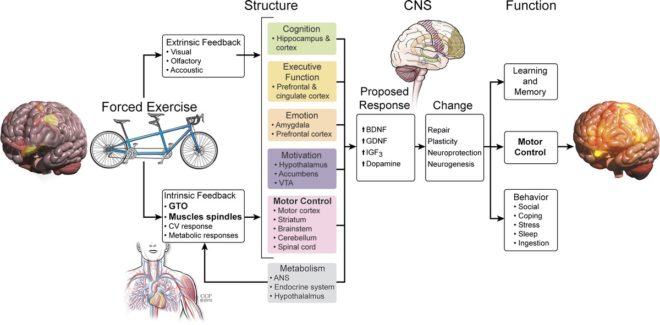
The aim of this review is to provide a brief overview of what is known about the effects of aerobic exercise training on the symptoms and motor function in patients with Parkinson’s disease (PD) and to detail the impact of a relatively new approach to exercise in human patients with PD, forced exercise (FE). FE, in this case, is defined operationally as a mode of aerobic exercise in which exercise rate is augmented mechanically to assist the participant in achieving and maintaining an exercise rate that is greater than their preferred voluntary rate of exercise. It is important to note that during FE, the participant is contributing actively to the exercise; they are not being moved through the motion passively. Our data indicate that FE leads to a global improvement in PD motor function and an alteration in the CNS function (22). These global changes in motor function and altered activation patterns provide strong evidence for the hypothesis that for patients with PD to derive motor benefits from exercise, assistance is required to achieve a rate of exercise that triggers the release of neurotrophic factors or possibly dopamine.
PD is a progressive neurodegenerative disorder affecting nearly 1.5 million Americans, with annual treatment costs approaching $25 billion. It is caused by selective neuronal loss in the substantia nigra and resultant degeneration of dopaminergic pathways in the basal ganglia. This loss of dopamine alters both inhibitory and excitatory pathways, resulting in its cardinal motor signs: bradykinesia (slowness of movement), tremor, rigidity, and postural instability (12). PD impacts movement ability, function, cognition, and quality of life (QOL), all to varying degrees on an individual patient basis. Traditional medical and surgical approaches to managing PD are expensive and associated with a variety of side effects that may further compromise QOL. Utilization of a non-drug, non-surgical therapeutic approach, such as exercise, to improve motor function would provide an attractive adjunct to current PD treatment approaches.